Radicals are reactive species that usually and readily abstract a hydrogen atom from metal hydrides. In some cases, in particular with substrates that meet certain geometric requirements, intramolecular C-H abstraction can take place. In this way, a new radical can be generated at an unactivated position, thereby allowing the introduction of functional groups at this position. The geometrical requirements dictate that the most frequently observed intra-molecular hydrogen transfers are 1,5-shifts, corresponding to specific attack on a hydrogen atom attached to a C-atom 5 from the initial radical. As shown below in the general example, hemolytic cleavage of the Y-X bond gives a radical Y* (normally nitrogen or oxygen centered), which is followed by hydrogen atom transfer. The resulting (more stable) carbon radical reacts with a neutral molecule or with a radical X’* which may or may not be identical with X*
One such example is the Hofmann–Loffler–Freytag reaction, which provides a method for the synthesis of pyrrolidines from N-halogenated amines. The reaction is effected by warming a solution of the halogenated amine in strong acid (e.g. H2SO4 or CF3COOH), or by irradiation of the acid solution with ultra-violet light. The initial product of the reaction is the δ-halogenated amine, but this is not generally isolated, and by basification of the reaction mixture it is converted directly to the pyrrolidine (or its derivatives). Both N-bromo- and N-chloro-amines have been used as substrates, although the N-chloro-amines usually give slightly better yields. The N-chloro-amines can be obtained from the amines by the action of sodium hypochlorite or N-chlorosuccinimide.
Thermal or photochemical dissociation of the N-chloro-ammonium salt, formed by protonation of the N-chloro-amine, is thought to give the reactive ammonium radical species. This abstracts a suitably situated hydrogen atom to give the corresponding carbon radical. This in turn abstracts a chlorine atom from another molecule of the N-chloro-ammonium salt, thus propagating the chain and at the same time forming the δ-chloro amine, from which the cyclic amine is obtained.
The mechanism is shown below:
(1). The Initiation Steps:
(2). The propagation steps:
(3). The final work up steps:
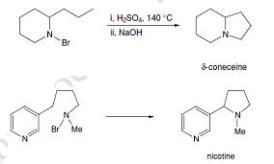
The first example of this type of reaction was reported by Hofmann in 1883. In the course of a study of the reactions of N-bromo-amides and N-bromo-amines, he treated N-bromo-coniine with hot sulfuric acid and obtained, after basification, a tertiary base that was later identified as δ-coneceine. Further examples of the reaction were reported later by Loffler, including a synthesis of the alkaloid nicotine. Many other cyclizations leading to simple pyrrolidines and to more complex polycyclic structures have since been reported.
The radical nature of the reaction is supported by a number of factors, including the fact that the reaction does not proceed in the dark at room temperature and that it is initiated by heat, light or iron (II) salts and inhibited by oxygen. The hydrogen abstraction step must be intramolecular in order to explain the specificity of reaction at the δ-carbon atom.
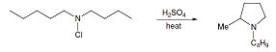
As with other radical reactions, secondary hydrogen atoms react more readily than primary as the resulting secondary radical is more stable. Thus, in the example given below, in the reaction of N-chloro-amine, attack by the nitrogen-centred radical on the δ-methyl group would lead to N-pentylpyrrolidine, whereas attack on the δꞌ-methylene would result in the formation of N-butyl-2-methylpyrrolidine. Only the latter compound was formed. Tertiary hydrogen atoms react very readily, but the resulting tertiary halides do not normally proceed to give cyclic amine products.
Some of the illustrative examples of Hofmann-Loeffler-Freytag reaction are shown below:
(1). An application of the Hofmann–Loffler–Freytag reaction is found in the synthesis of the steroidal alkaloid derivative dihydro-conessine which is as shown below. In this synthesis, the pyrrolidine ring is constructed by attack on the unactivated C-18 angular methyl group of the precursor by a suitably placed nitrogen radical. The ease of this reaction is a result of the fact that in the rigid steroid framework, the C-18 angular methyl group and C-20 side chain carrying the nitrogen radical are suitably disposed in space to allow easy formation of the six-membered transition state necessary for 1,5-hydrogen atom transfer.
(2). When haloamines are the derivatives of primary amines, reaction takes place in the presence of Fe(II) as the initiator.
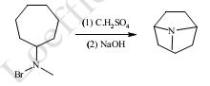
(3). With N-halocycloalkylamines cyclization leads to bridged ring structures, but in these cases products may not be exclusively pyrrolidine derivatives. In some cases, six membered bicyclic ring is formed instead of five membered heterocyclic ring. N-Bromo-N-methyl cycloheptylamine gives tropane. In this case pyrrolidine ring is formed.
References:
(1). Modern Methods of Organic synthesis by William Carruthers & Iain Coldham; Cambridge University Press.
(2). Photochemistry and Pericyclic Reactions by Jagdamba Singh and Jaya Singh; New Age International Publishers.
(3). M. E. Wolff, Chem. Rev., 63, 55 (1963); “Cyclization of N-Halogenated Amines (The Hofmann-Loffler Reaction)”